Topic
Cold Seeps

Ocean Fact: What are gas hydrates?
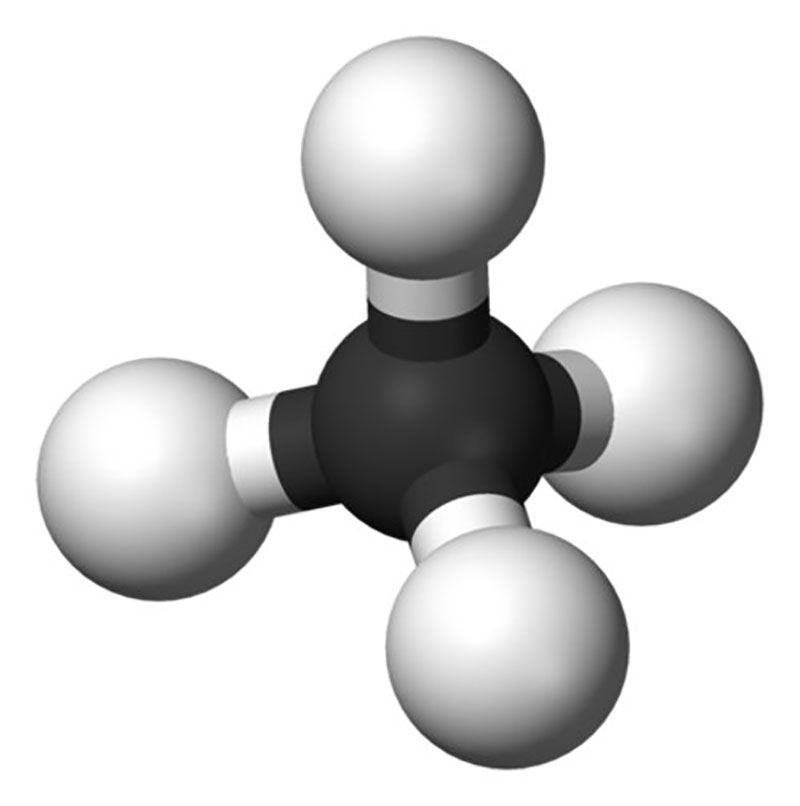
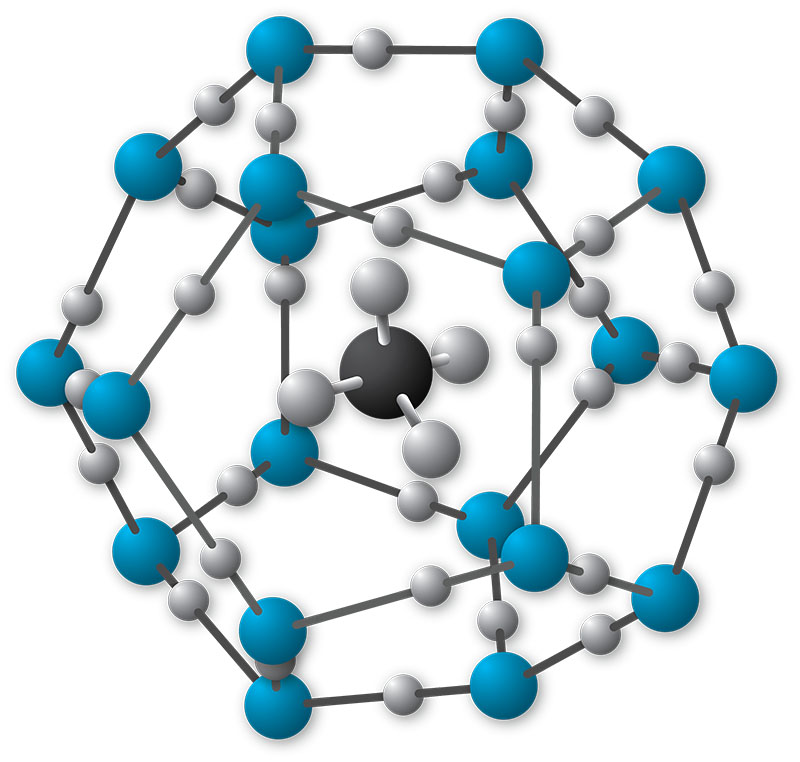
Gas hydrates are ice-like crystalline structures that form when a low-density gas, like methane, ethane, or carbon dioxide, combines but does not chemically bond with water and freezes into a solid under low temperature and moderate pressure conditions. Most gas hydrates are formed from methane (CH4), which has led to the terms “gas hydrate” and “methane hydrate” often being used interchangeably.
On Earth, gas hydrates occur naturally in some marine sediments and within and beneath permafrost. They are also speculated to form on other planets.
For us, hydrate deposits are important for a variety of reasons:
- Gas hydrate deposits may contain roughly twice the carbon contained in all reserves of coal, oil, and conventional natural gas combined, making them a potentially valuable energy resource.
- Their decomposition can release large amounts of methane, which is a greenhouse gas that could impact Earth’s climate.
- Sudden release of pressurized methane gas may cause submarine landslides, which in turn can trigger tsunamis.
- Gas hydrates in the ocean can be associated with unusual and possibly unique biological communities that use hydrocarbons or hydrogen sulfide for carbon and energy, via a process known as chemosynthesis.